The FDA's DSCSA Deadline is Coming Up: Are You Ready?
The FDA’s Drug Supply Chain Security Act (DSCSA) deadline is just around the corner, on November 27, 2023. This law mandates that a number of security measures be implemented by all drug manufacturers, repackagers, wholesale distributors, and 3rd Party Logistics companies to help prevent contaminated and adulterated drugs from entering the supply chain.
If you are not yet compliant with the DSCSA, you must start doing so right now. There are many tools at your disposal to assist you in DSCSA compliance. These include:
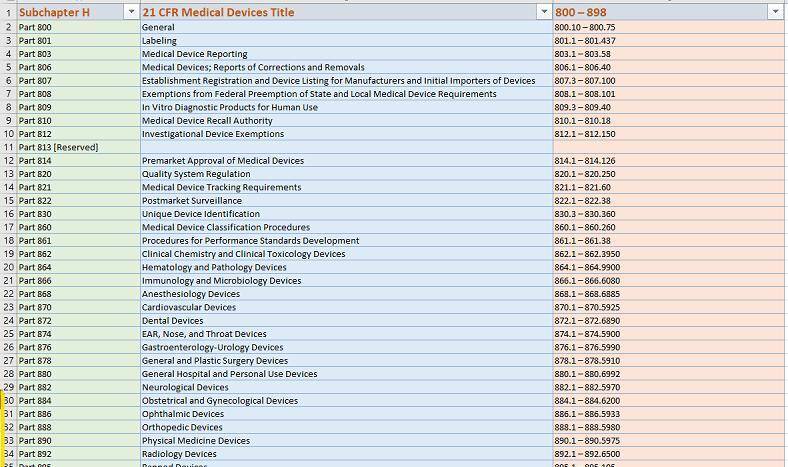
- The FDA’s website: The FDA has a dedicated PDF Database for its users which includes a wealth of information. You can also visit the US FDA to search for ‘DSCSA’ guidance documents.
- The Healthcare Distribution Alliance: The Healthcare Distribution Alliance offer a number of resources to help their members including training, webinars, and a compliance toolkit. Their boot camp style seminar – happening in Washington DC can help get familiar with DSCSA. Click here to signup. Link
- Partnership for DSCSA Governance: Partnership for DSCSA Governance (PDG) is an independent and Non-Profit organization that works to prevent harmful drugs from entering the supply chain.
Regardless of the resource you choose to learn about DSCSA, it is an undeniable fact that you will need to spend a lot of time. This is where our tool SmartSearch+ comes in handy for all drug manufacturers, repackagers, wholesale distributors, and 3rd Party Logistics companies.
With SmartSearch+, you can search the full text of all FDA/Gov guidance documents, including those related to the DSCSA so that you can meet the deadline. So, request completely Free access to SmartSearch+ by clicking the link below. No credit card is required.
See the link to know more about how SmartSearch+ is a game-changer: Click Here
Here are some additional tips for complying with the DSCSA:
Start Early: There is a lot to do to comply with the DSCSA. Create a plan and track your progress. This will help you stay on track and avoid making mistakes. Use the resources available to you. SmartSearch+ is a great tool to help you get started.
Get help from a qualified professional: If you need help, feel free to reach out to us for professional assistance or consultancy.