About eSTARHelper
eSTARHelper LLC is a unique hybrid, FDA regulatory and Microsoft Partner consulting company. In a nutshell, here’s out story
eSTARHelper was spawned by recognizing the new normal of today’s medical device companies undisputedly operating in hybrid mode with some on-site, some at-home remote work force. eSTARHelper inherited its parent company FDASmart biopharma and medical device regulatory legacy and experience and merged its eSTARHelper Microsoft Partner company prowess into ONE COMPANY.
The end result of this part- FDA regulatory consultancy /part Microsoft-IT Partner fusion uniquely sets us apart in today’s medical device world overwhelmed with the creation and management of medical device processes which include but not limited to–design, development, risk management, quality systems and remediation mandatory requirements and many others that demand the accurate and time management of a data ‘firehose’.
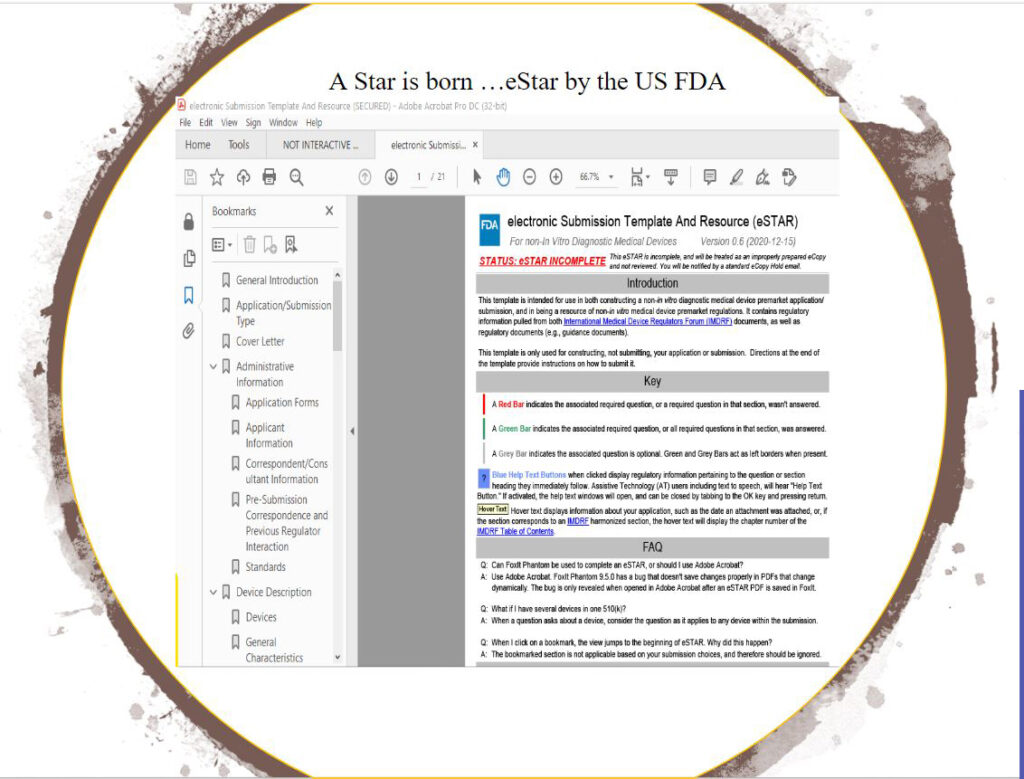
US FDA’s CDRH (Center for Device and Radiological Health), the overseers for medical device premarket notifications are on the verge of a digital transformation to catch up with secure cloud-driven systems that most large medtechs have adopted earlier though commanding a hefty price tag for proprietary hosting or software licenses. CDRH recently issued eSTAR, its 510(k) Premarket Submission PDF template and expect to promote its use to become mandatory in time in order to achieve its MDUFA IV deliverable goals of 60-day 510K reviews.
eSTAR from FDA, lacks features that CDRH leaves to the submitter or 510K applicant yet expects 21 CFRs regulatory compliance for. Among them are:
❌
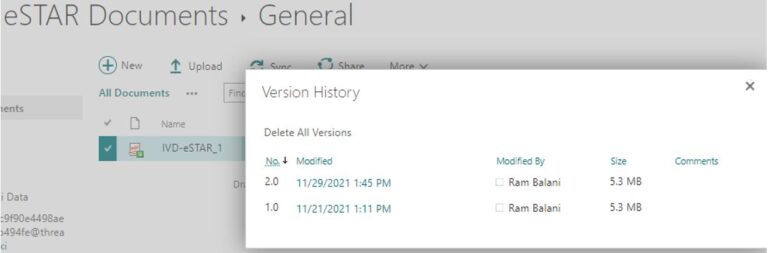
Lack of inherent 21 CFR Part 11 Version control for the actual eSTAR .PDF template
✔
eSTARHelper Fix – Microsoft TEAMs —provide 21 CFR Part 11 compliant version control on secure and authenticated cloud hosted copy of eSTAR after FDA site download
❌

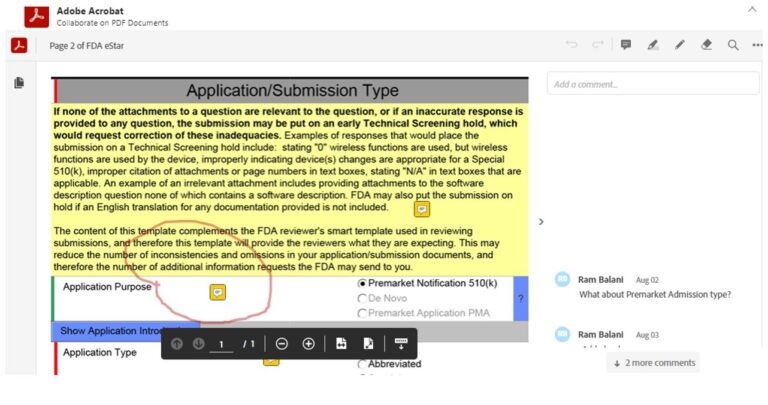
No ability to post customary Adobe PDF page notes, highlighting or pen-drawing for sharing comments and collaboration
✔
eSTARHelper Fix – Microsoft TEAMs — pre-created Channels in Teams from flat-printed eSTAR PDF pages, each eSTAR PDF page extracted becomes its own Channel in Teams with ‘Posts’, ‘Files, & extensible additional new ‘Tabs’
❌

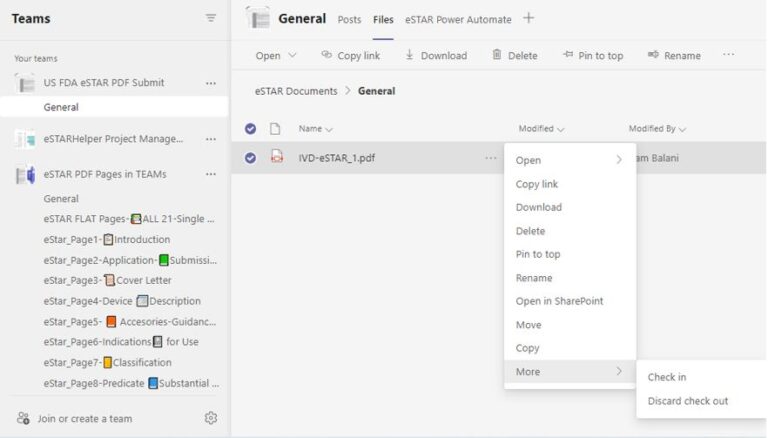
No way to share and synchronize contents of multiple copies of eSTAR PDF, i.e. though eSTAR allows Export/Import of the file contents via XML, problem is combining several copies with disparate contents are not possible (i.e. XML Import is a ‘replace’ not ‘append’ to an anchor or master eSTAR PDF copy). Multiple eSTAR copies each with its incomplete share of eSTAR data can be floating around!
✔
eSTARHelper Fix – Microsoft TEAMs — disallows multiple copies of eSTAR in the secure, encrypted and fully authenticated Microsoft Cloud tenant site that forces Check-Out/Check In protocols; each previous version still available for viewing despite overriden by more recent saves
❌

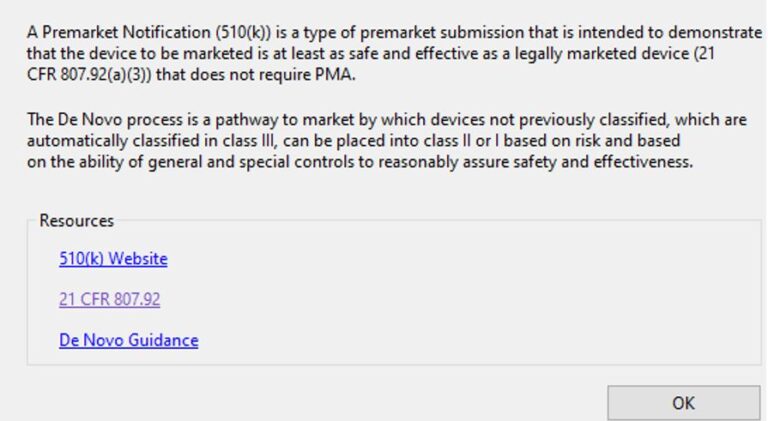
Limited Help screens on eSTAR pages redirects to US FDA CFR website data where no intelligent search is
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?FR=807.92
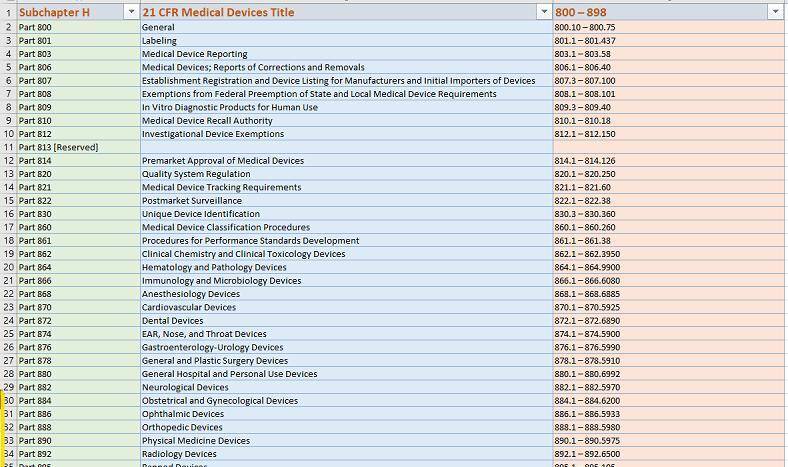
eSTARHelper Fix – Microsoft TEAMs — SmartSearch from eSTARHelper to the rescue, i.e. we’ve compiled (* not replaced*) all thrity-four CFRs that are part of FDA Subchapter H for medcial devices, hosted online and made each CFR (e.g. 21 CFR 820 – Quality Systems) SmartSearchable with Google-like keywords but without the Internet search clutter on US FDA website.
Additionally-eSTARHelper makes available customized beyond OOTB (Out of the box) Sharepoint features that allow for post-search (on search results) specific follow-on actions like – one-click Channel Post Message or Planner Task auto-creation in Teams.
As an example, our in-house SmartSearch on Sharepoint cloud, when used for your benefit, allows us to conduct a rich, intelligent search in 21 CFR Part 820 (Quality Systems) without the Internet clutter from Google and superior to US FDA website own search engines.
The same applies to ALL Other 21 CFRs on medical devices (US FDA Subchapter ‘H” – 34 CFRs in all)
See sample 21 CFR Part 820 Search for any statute referencing ‘quality’ ‘supplier’ as shown below:
(* Challenge: NOW – Try to search for “quality” “supplier” at FDA website for BOTH KEYWORDS referenced by the same statute *)
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1
A picture is worth a thousand words they say—see the eSTARHelper demo video recording here
In today’s always-on, big data and social media for business driven paradigm, the old school approaches of regulatory compliance by medical device companies will no longer work. Chasing emails and stand-alone Word docs, Excel sheets or databases stored all across disparate platforms and application systems no longer work !
The medical device industry is compromised mostly of companies with fewer than 100 people employed, perhaps a number of outsourcing partner and business alliances formed with external parties. Quality management systems of today are a one size built for all generic offerings where medtechs are forced to work around vendor platforms and services instead of the other way around. A vendor to vendor offering systems customized for each medical device company is clearly not feasible and would be astronomical in costs if it were.
Enter Microsoft with its Microsoft Cloud technology and its omnipotent presence with Office 365/Sharepoint and Teams platform. Chances are any medical device company, big or small, already use one or ALL of these ever-popular Microsoft application platforms.
eSTARHelper is uniquely positioned to assist with new or evolving deployment of your in-house Office 365/Sharepoint or even Teams platforms in the light of US FDA CDRH eSTAR and all medical regulatory compliance mandates.
Around 50% of Microsoft 365’s 260 million+ commercial users now use Teams.
Here below is a sample use case of why Microsoft Teams has become the ‘darling’ of collaboration software platforms world over. NHS requested a consortium in the UK be mobilized post haste to produce in unprecedented time and number of ventilator units spawned by the dire need for ventilator shortages caused by Covid-19.
The largely remote UK-based consortium members had to manage meetings, files exchanges including NDA for IP protection, design and manufacturing collaboration and much more while achieving impressive results in record time ALL remotely synchronizing work schedules, data and workflow processes, messages and emails in real-time.
Here’s what one consortium partner had to say about Microsoft Teams:
Microsoft Teams
Its astounding to think that for several weeks of the ventilator projects many of had not met face-to-face and only over the virtual medium of Microsoft Teams. Its been a rapid education in how it can make our teams more effective and efficient and we see the positive impact it can have on our businesses going forward
Mark Mathieson, Lead Partner Technology Service, McLaren Racing
What is SharePoint – Microsoft Teams relies on Sharepoint Online for its backend platform and services.
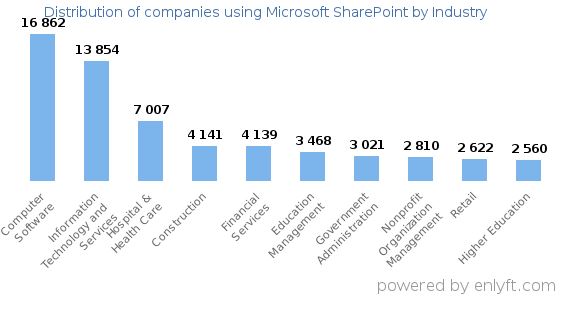
Top Industries that use SharePoint