
SMARTSEARCH FDA TOWNHALLS
SMARTSEARCH 21 CFR 820 - QUALITY SYSTEMS
COVID 19 GUIDANCE - MEDICAL DEVICES SMARTSEARCH
SMARTSEARCH 21 CFR 803- MEDICAL DEVICE REPORTING
SMARTSEARCH 105 MEDICAL DEVICE 510K GUIDANCE





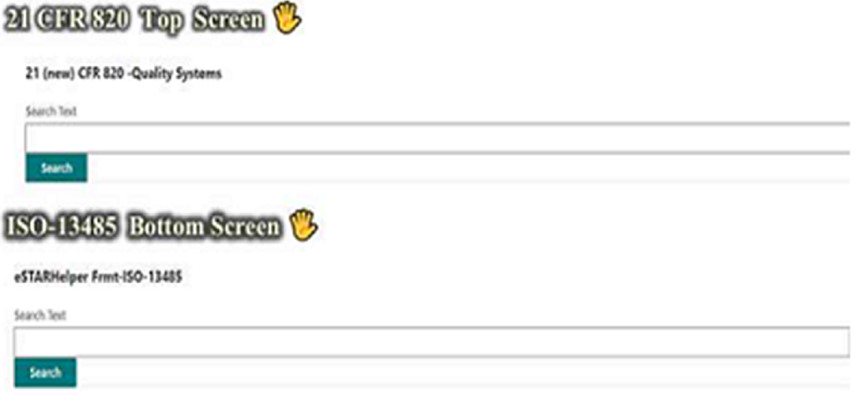

SmartSearch in one screen – 21 CFR 820 Quality Systems vs ISO-13485 Standard
Get SmartSearch FREE , No Credit Card Access
How eSTARHelper SmartSearch Works
🚀 SmartSearch is a compilation of content that enables full-text keyword searching of:
📚 US FDA website Medical Device CFRs (Code of Federal Regulations, e.g. 21 CFR 820 Quality Systems)
e.g. find US FDA CFR 820 statutes on ‘quality’ NOT ‘supplier’
📚 US FDA’s (ALL) PDF Guidance content on Medical Services
e.g find US FDA PDF Guidance on ‘sterile’ next to ‘cardiovascular’
📚 ISO-13485 Standard Clauses for Quality Systems with Compliance Document Toolkits Free sample view linked to MATCHING search-selected ISO-13485 standards clauses
e.g. find ISO-13485 Standards clauses on ‘quality’ ‘supplier’
📚 US FDA Covid-19 Virtual Town Hall transcript recordings
e.g. find ‘saliva’ NEAR ‘home’
📚 Soon.. all Subchapter ‘H’ US FDA CFRs on Medical Devices (*34 total CFRs *)
📚 Soon.. other ISO medical device standards, i.e. ISO-14971

