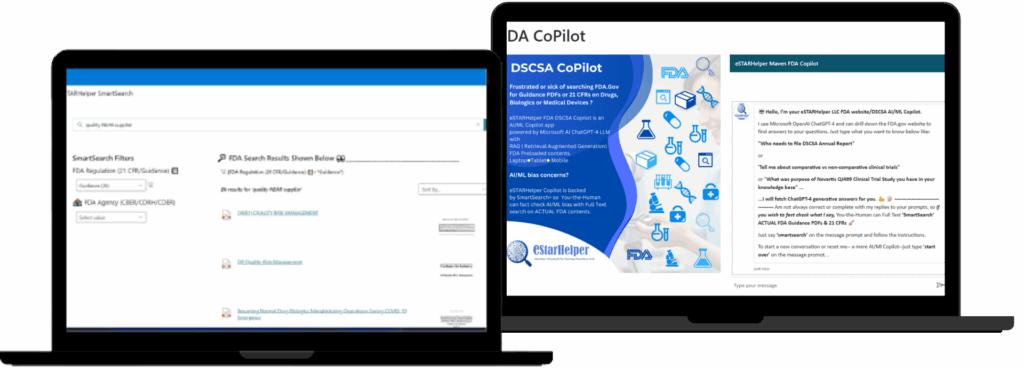
AI-Powered FDA Compliance at Your Fingertips
Accelerate regulatory clarity with our all-in-one app—featuring Microsoft ChatGPT-4 Copilot, SmartSearch+, and secure enterprise integration.

Our ‘Dueling Banjo’ strategy is one of a kind’
Users can ChatGPT-40 converse with the FDA
Website (FDA.Gov) in Natural Language when
www.estarhelper.com/FDACopilot is launched
BUT -at any time- user can enter ‘smartsearch’ (* no quotes)
You-the-Human can full text ALL Guidance PDFs & 21 CFRs
On Drugs, Biologics and Medical Devices from one app screen
On laptops, tablets or mobiles.
THE PROBLEM WE SOLVE
Regulatory compliance shouldn’t feel like drinking from a firehose. FDA and SFDA guidelines are complex, fragmented, and constantly changing. Traditional search methods are slow, outdated, and error-prone.

Searching FDA.gov or Google is hit-and-miss

Guidance PDFs and CFRs are buried in silos

Risk of non-compliance costs time, money, and reputation
OUR SOLUTION
THE TRIFECTA OF INNOVATION

AI/ML Copilot
Generative ChatGPT-4 bot, trained on FDA and SFDA content, powered by Microsoft Azure
Proprietary Data Grounding
Securely integrates with Microsoft 365, SharePoint Online, and Teams for enterprise-specific answers

SmartSearch+
Back-end full-text search engine to verify AI answers using original PDFs and guidance docs

Our AI/ML-powered regulatory Copilot is built for:
- Regulatory Affairs & QA Professionals
- Biopharma & Medical Device Manufacturers
- CROs, CDMOs, & GxP Training Providers
- Legal & Compliance Advisors
- Government Agencies (e.g. FDA, SFDA, ICH)
- VC/Investors evaluating life science tech
TRUST STATEMENT
A powerful solution to navigate the complexity of FDA guidelines with confidence. It’s like having a 24/7 regulatory advisor.

Former Deputy Director
U.S. FDA (Board Member)