SmartSearch+ Tab on Microsoft Teams to perform 21 CFRs level search on:
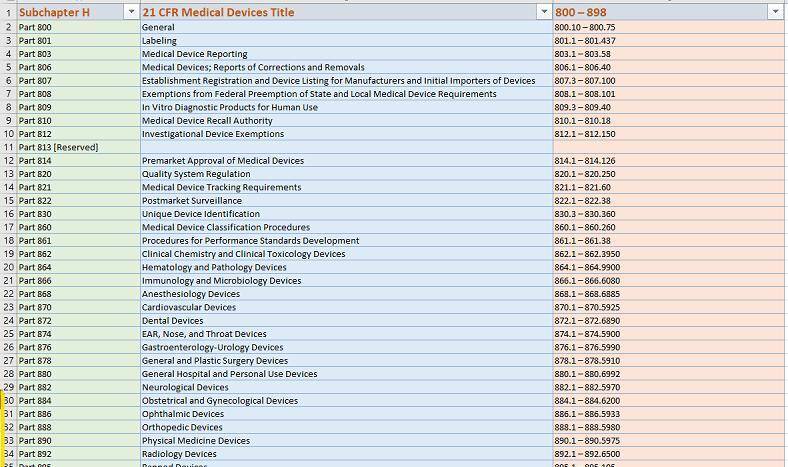
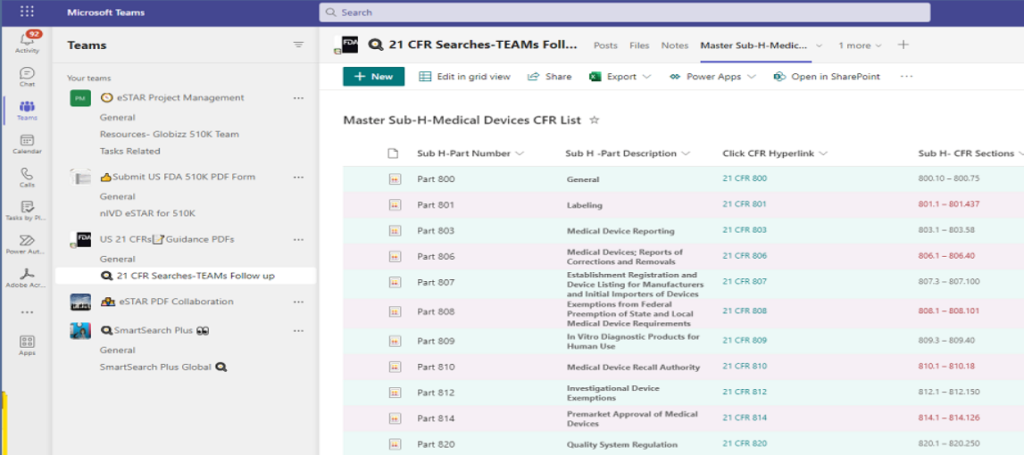
📚 All 21 CFRs for Medical Devices ( Subchapter H -34 Total)– by CDRH– Center for Devices & Radiological Health
📚 All 21 CFRs for Biologics ( Subchapter F- 11 Total) – by CBER – Center for Biologics & Evaluation Research
📚 All 21 CFRs for Drugs ( Subchapter C * D -51 Total) – by CDER – Center for Drug Evaluation & Research
🍳 Microsoft Teams integration with SmartSearch+
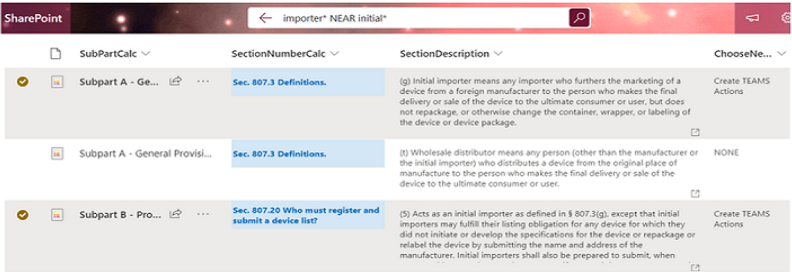
Step 1:
User SmartSearch+ on 21 CFR 807 for ‘importer’
references to ‘initial’.
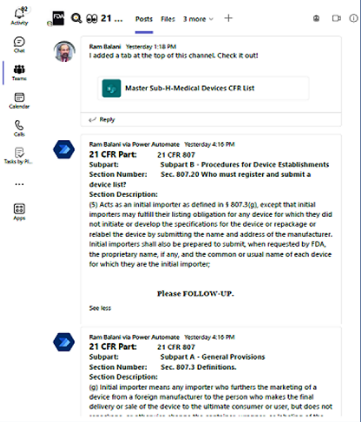
User flags 1st sea & 3rd row results as candidates for Microsoft Teams channel messaging .
Each rows flagged auto-generates a Teams Message in a channel selected below.
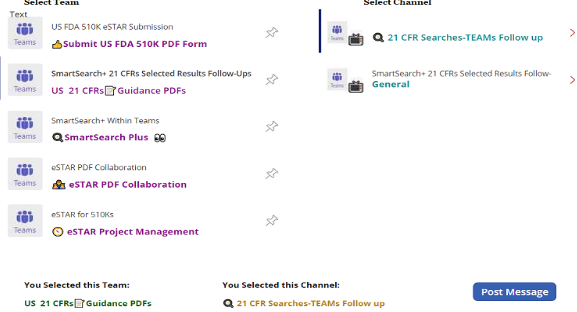
Step 2:
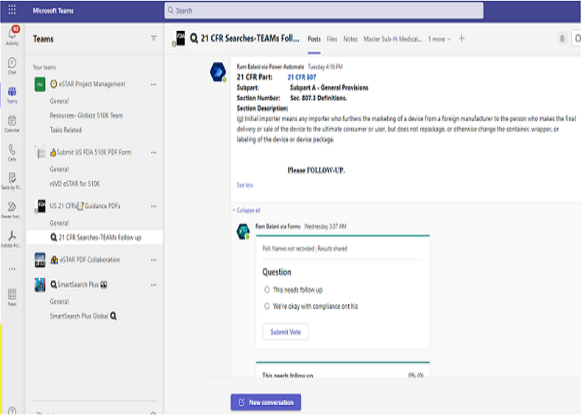
User is shown local enterprise Teams environment and each team’s channels.
User pick a specific team and specific channel within chosen team to earmark where Teams Post Message is published.
Teams posted message shows 21 CFR 807 Subpart, Scope, Section Number and Section Description. See next slide for sample.
SmartSearch+ -auto-generates with one (1) click Microsoft Teams messages from one or many SmartSearch+ results context on your selected Teams/Channel FDA results data -ALL 21 CFR Subpart, Scope, Section No & Section Description search text in channel posted messages ready for MORE out-of-the box Teams features, e.g. tasks,chats – no chasing email