About FDA eSTAR
What is eSTAR?
On September 29, 2021, the FDA issued a draft guidance: Electronic Submission Template for Medical Device 510k Submissions. The guidance outlines the structure, format, and use of the electronic Submission Template And Resource (eSTAR) for preparing electronic 510(k) submissions
The electronic Submission Template And Resource (eSTAR) is a PDF electronic submission template that guides premarket notification (510(k)) submitters through the process of preparing a comprehensive medical device 510k submission. This template contains:
- Automation (for example, form construction, autofilling);
- Content and structure that is complementary to CDRH internal review templates;
- Integration of multiple resources (for example, guidances, databases);
- Guided construction for each submission section; and
- Automatic verification (i.e., FDA does not intend to conduct a Refuse to Accept review)
- Internal eSTAR form-filling tracking and marked ‘Complete’ when fully compliant
- Blue Help/Notification Screens, Consensus Standards Drop-downs list are context specific
The voluntary eSTAR pilot program aims to improve consistency and efficiency in how the medical device industry prepares 510(k)s and how the FDA reviews these submissions. The FDA will evaluate whether the use of FDA’s free eSTAR produces well-organized submissions that can be reviewed more efficiently, in comparison to submissions prepared as eCopies or with the eSubmitter application, to help promote timely access to safe, effective, and high-quality medical devices.
At the moment, eSTAR can be used on a voluntary basis to construct a 510k submission. The date for mandatory use of eSTAR for 510k will be identified when the draft guidance is finalized. While eSTAR is currently only available for 510k submissions, the FDA intends to expand the eSTAR program to include other types of pre-market and post-market medical device submissions in the future.
❌ What’s NOT POSSIBLE with FDA eSTAR PDF template after download from US FDA website……❌
📝 Downloaded FDA eSTAR PDF template pages can’t be annotated with Adobe highlighting, annotation or comments added for collaboration and sharing (* unless PDF flat-printed each*)
🙏 FDA eSTAR PDF (.PDF file submitted to US FDA) template does not have embedded 21 CFR Part 11 version control
🍳 eSTAR attachments added by medical device 510K applicant need to maintain external 21 CFR Part 11 regulatory compliance, i.e. audit trails, security, data accuracy up to medical device Sponsor t enforce
⛳ Blue button Help Screens are context sensitive per eSTAR sections but limited, e.g. Help link redirecting to 21 CFR Part 807 for example can’t be searched intelligently on FDA website CFR home , i.e. find ‘Importer’ ‘Not initial’
🛩 FDA eSTAR Export/Import limited -XML import is ‘Replace’ only not ‘Append’ – all attachments to shared partial eSTAR PDF Pages are lost (* orphaned*)
eSTARHelper to the rescue ….see MORE DETAILS on how eSTARHelper FIXES
FDA eSTAR deficiencies when the PDF template is hosted in your secure, authenticated Microsoft Teams/Sharepoint site and how eSTARHelper 🚀turbo-charges FDA CDRH’s downloadable eSTAR PDF template here.
👀 FDA’s eSTAR PDF hosted online integrates well into Microsoft TEAMs environment – ever so relevant in today’s new normal, onsite & virtual global workforce hybrid environment
🚀 Microsoft TEAMs out-of-the-box (OOTB) & advanced customizable Office 365/Sharepoint features turbo-charges eSTAR with ‘embrace & extend’ paradigm. e.g.
Enables eSTAR PDF 21 CFR Part 11 version control and associated electronic document attachments with out-of-the-box Sharepoint secure audit trails and comprehensive Microsoft Compliance Center ad-hoc or scheduled reporting
👍 eSTAR flat-printed PDF pages when hosted in Teams channels, enhances sharing and collaboration with Adobe tools such as highlighting, text annotations even pen-drawn notes
✔ Office 365/Sharepoint approval request and approval workflows enables eSTAR document attachments management control and processing complete with enhanced with email alerts or Teams channel messages
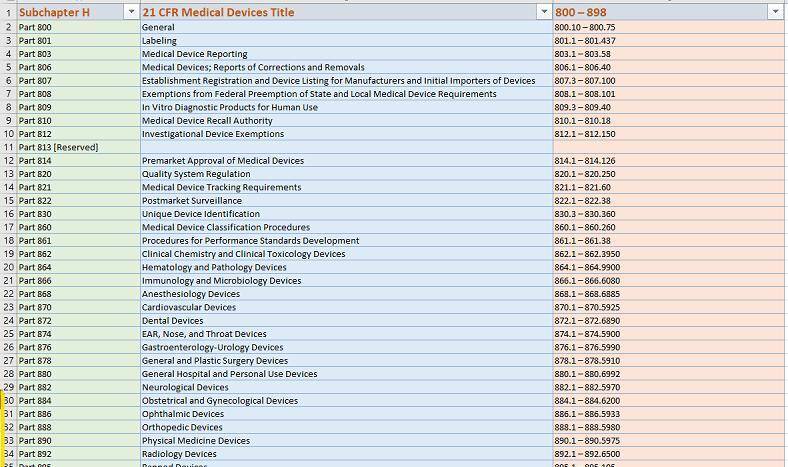
Here’s what eSTAR PDF template for 510k looks like below:
Page 1

Page 2
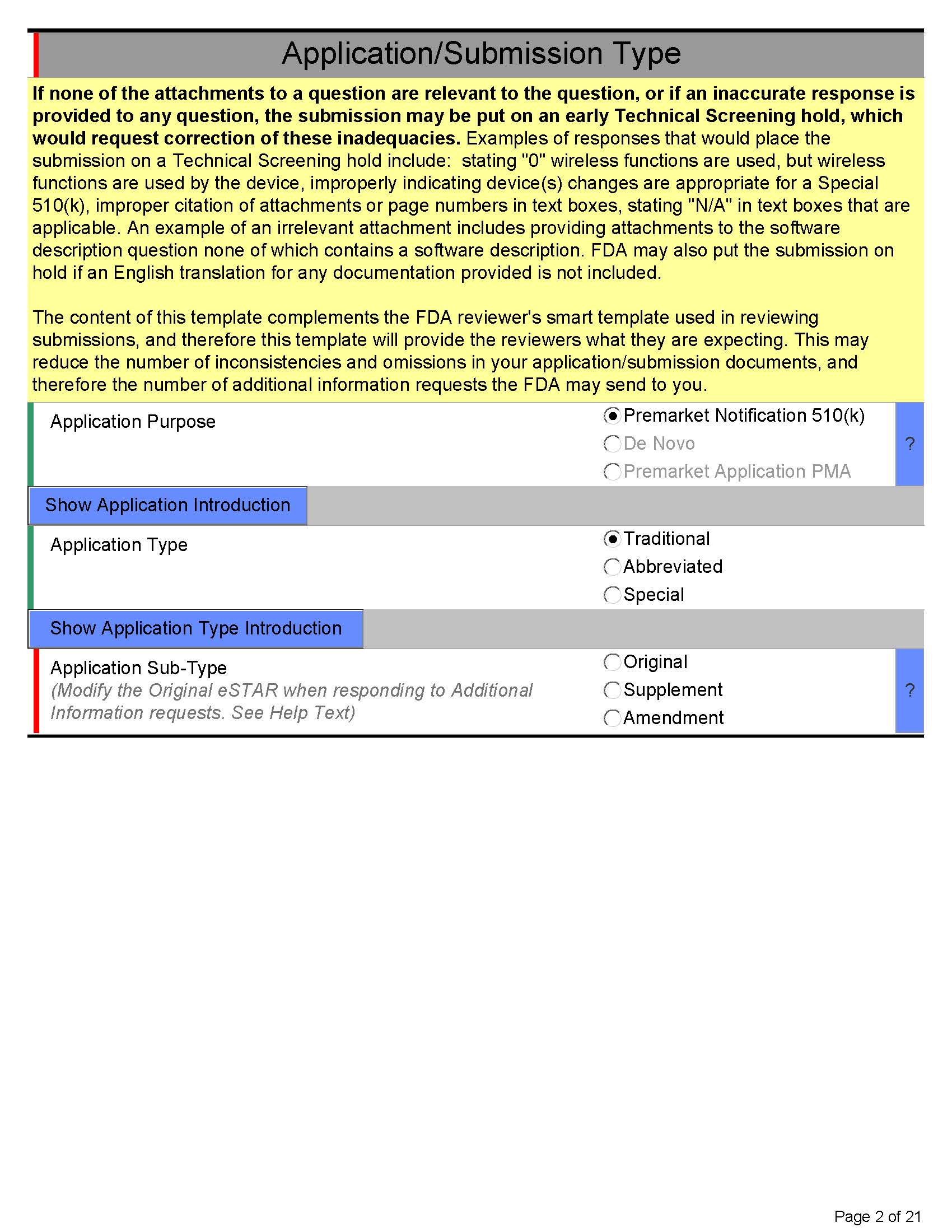
Page 3
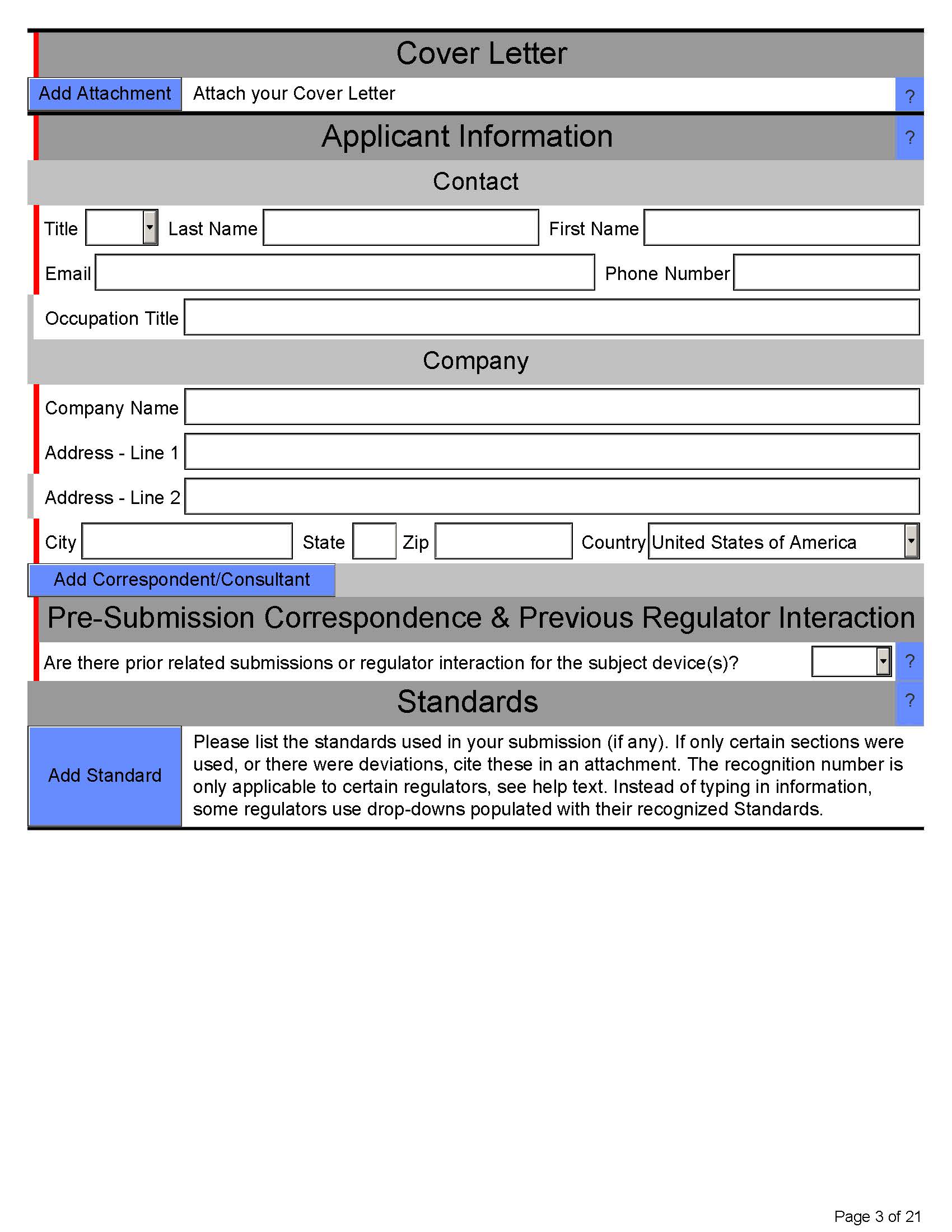
Page 4
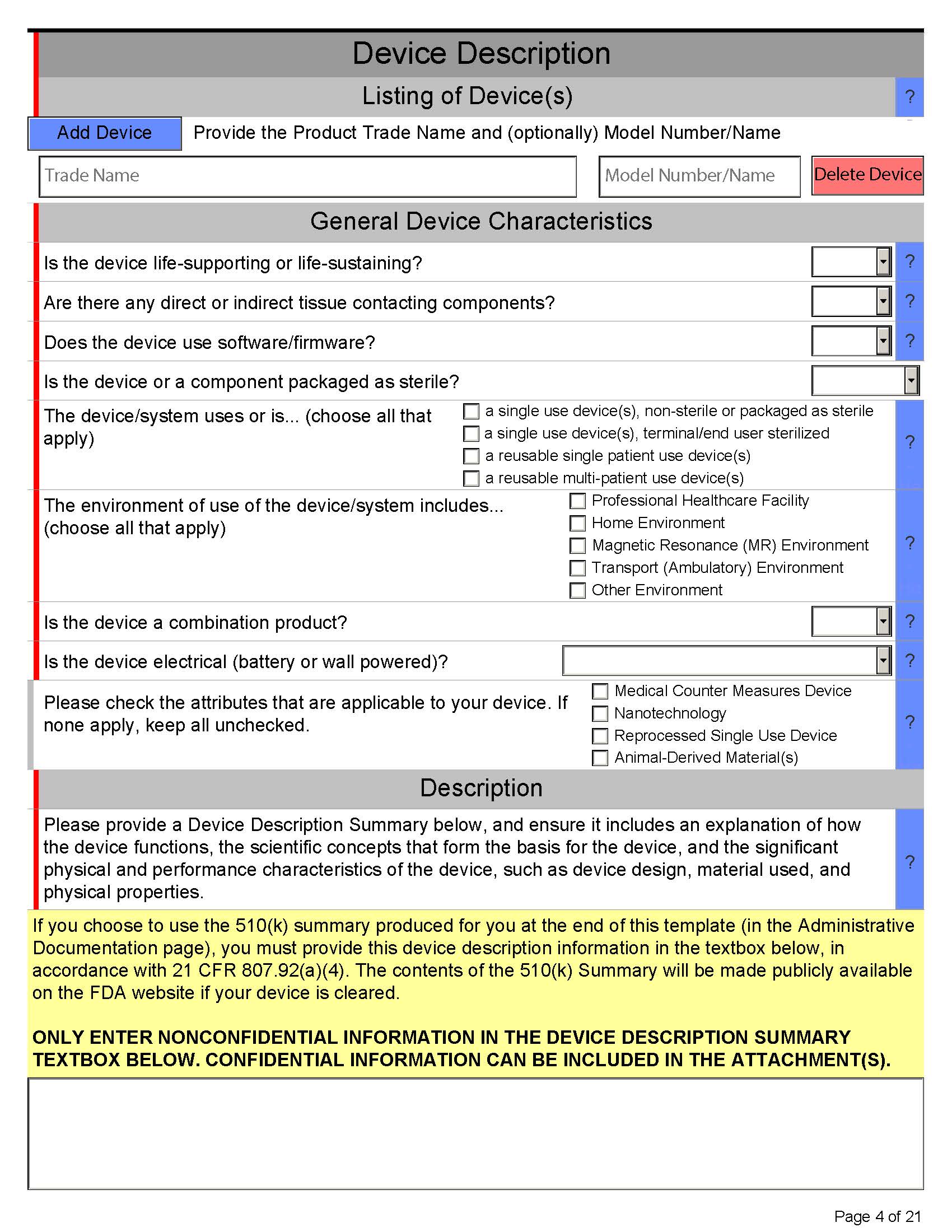
Page 5

Page 6

Page 7
Page 8

Page 9

Page 10
Page 11

Page 12
Page 13

Page 14
Page 15
Page 16
Page 17

Page 18

Page 19

Page 20

Page 21